Quality Document and Process Management for CAPAs, Deviations and Change Controls
How we help
Agatha QMS Benefits
-
 Regulatory Compliance and Streamlined Inspection ReadinessAgatha ensures compliance with regulatory standards such as 21 CFR Part 11, ISO 13485, GMP, and GCP, while centralizing deviation management, change control, quality information, and CAPA (Corrective and Preventive Actions). This facilitates smooth audit responses and reduces operational burdens.
Regulatory Compliance and Streamlined Inspection ReadinessAgatha ensures compliance with regulatory standards such as 21 CFR Part 11, ISO 13485, GMP, and GCP, while centralizing deviation management, change control, quality information, and CAPA (Corrective and Preventive Actions). This facilitates smooth audit responses and reduces operational burdens. -
 Increased Productivity Through Systemization and Workflow AutomationWith version control, access management, and workflow automation, Agatha reduces manual tasks, prevents errors, and standardizes quality management operations, improving efficiency across the organization.
Increased Productivity Through Systemization and Workflow AutomationWith version control, access management, and workflow automation, Agatha reduces manual tasks, prevents errors, and standardizes quality management operations, improving efficiency across the organization. -
 Improved Risk Management and Data-Driven Quality EnhancementsReal-time quality data analysis enables early detection of potential issues, allowing for swift corrective actions. By optimizing deviation management, CAPA, and change control processes, Agatha minimizes quality risks and enhances overall compliance.
Improved Risk Management and Data-Driven Quality EnhancementsReal-time quality data analysis enables early detection of potential issues, allowing for swift corrective actions. By optimizing deviation management, CAPA, and change control processes, Agatha minimizes quality risks and enhances overall compliance. -
 Cost-Effective Implementation with Reduced Time and EffortBy eliminating the need for on-premise server management, Agatha reduces deployment and maintenance costs. Additionally, paperless operations lower printing and storage expenses, making quality management more cost-efficient.
Cost-Effective Implementation with Reduced Time and EffortBy eliminating the need for on-premise server management, Agatha reduces deployment and maintenance costs. Additionally, paperless operations lower printing and storage expenses, making quality management more cost-efficient. -
 Advanced Security and Flexible Access ControlOperating in a secure cloud environment, Agatha ensures that the latest security measures are always in place. Granular permission settings allow authorized personnel to access only the necessary information, safeguarding data integrity and confidentiality.
Advanced Security and Flexible Access ControlOperating in a secure cloud environment, Agatha ensures that the latest security measures are always in place. Granular permission settings allow authorized personnel to access only the necessary information, safeguarding data integrity and confidentiality.

NS Pharma improves its Quality and eTMF management with Agatha
Main Features
Work with ready-to-use standard forms and processes, or adapt them to support your needs. Use the form designer to update the look and framework of your forms.
A dynamic, multipart form supports all steps in a process from deviation to change control, adding appropriate sections based on information provided at each stage. Reviews, approvals, and assignments for actions are also built-in to help businesses reach their quality objectives.
Get all the reporting you need with built-in dashboards and reports, generate custom reports and export any report to Excel or a generic CSV format.
Cross Workspace Reporting: You can also create views and reports across workspaces and export results to Excel for dashboard reports.
Get complete quality records with signatures and audit trails, ready for inspection. Agatha QMS is fully compliant with 21 CFR Part 11 and EU regulatory requirements.
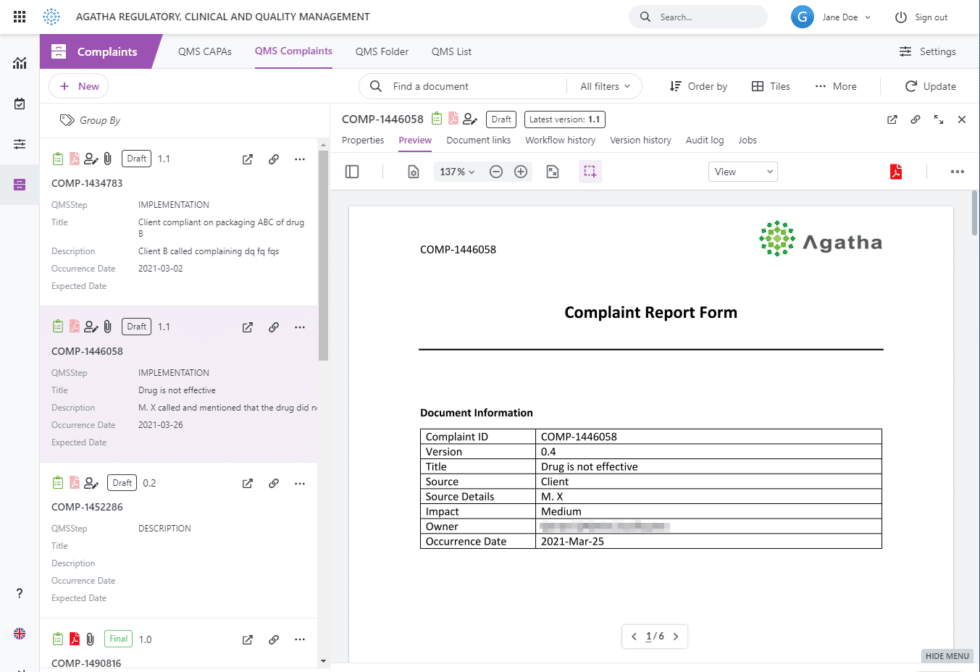
QMS forms in Agatha can be converted to PDF at any point, resulting in an appropriately-format, signed electronic record.
Ready do dive in?
For inquiries about our products and services, or to sign up for a demo, click here.