Management of Clinical Site Documents and Associated Processes
How we help
Agatha Remote eISF Benefits
-
 Elevate quality and cut down costs with Agatha’s remote ISF solutionPlanning to diminish time and expenses associated with the monitoring of your clinical trial sites? Our eISF solution presents a superior model, negating the need for on-site visits and reducing the administrative burden.
Elevate quality and cut down costs with Agatha’s remote ISF solutionPlanning to diminish time and expenses associated with the monitoring of your clinical trial sites? Our eISF solution presents a superior model, negating the need for on-site visits and reducing the administrative burden. -
 Bring uniformity to your clinical research sites with Agatha’s eISFUtilize Agatha’s eISF solution to simplify your clinical trial process, maintaining audit-ready essential study documents. Achieve remote access to all critical trial documentation and promptly identify missing records, ensuring a compliant conduct of your trials.
Bring uniformity to your clinical research sites with Agatha’s eISFUtilize Agatha’s eISF solution to simplify your clinical trial process, maintaining audit-ready essential study documents. Achieve remote access to all critical trial documentation and promptly identify missing records, ensuring a compliant conduct of your trials. -
 Facilitate effective sponsor systems interaction with remote monitoring via Agatha’s eISFAgatha’s eISF solution empowers monitors and inspectors to review source files, make notes, and complete their monitoring tasks, contributing to an efficient clinical trial process. The application also facilitates the creation and assignment of tasks for site administrators, enhancing the communication between technology systems.
Facilitate effective sponsor systems interaction with remote monitoring via Agatha’s eISFAgatha’s eISF solution empowers monitors and inspectors to review source files, make notes, and complete their monitoring tasks, contributing to an efficient clinical trial process. The application also facilitates the creation and assignment of tasks for site administrators, enhancing the communication between technology systems.

Medical Device Company, Optos, Amps up ClinOps with Agatha Applications
Main Features
Craft separate, protected areas for each Site to handle its documents. Each site and study incorporates a complete set of placeholders for expected eISF documents. Apply configurable auto naming to maintain naming conventions adhering to trial standards and facilitate compliant document management.
View all your workspaces for each clinical trial site in the dashboard and quickly see and access tasks assigned to you. Use the configurable views to inspect, verify, and identify gaps in the expected eTMF contract at any time.
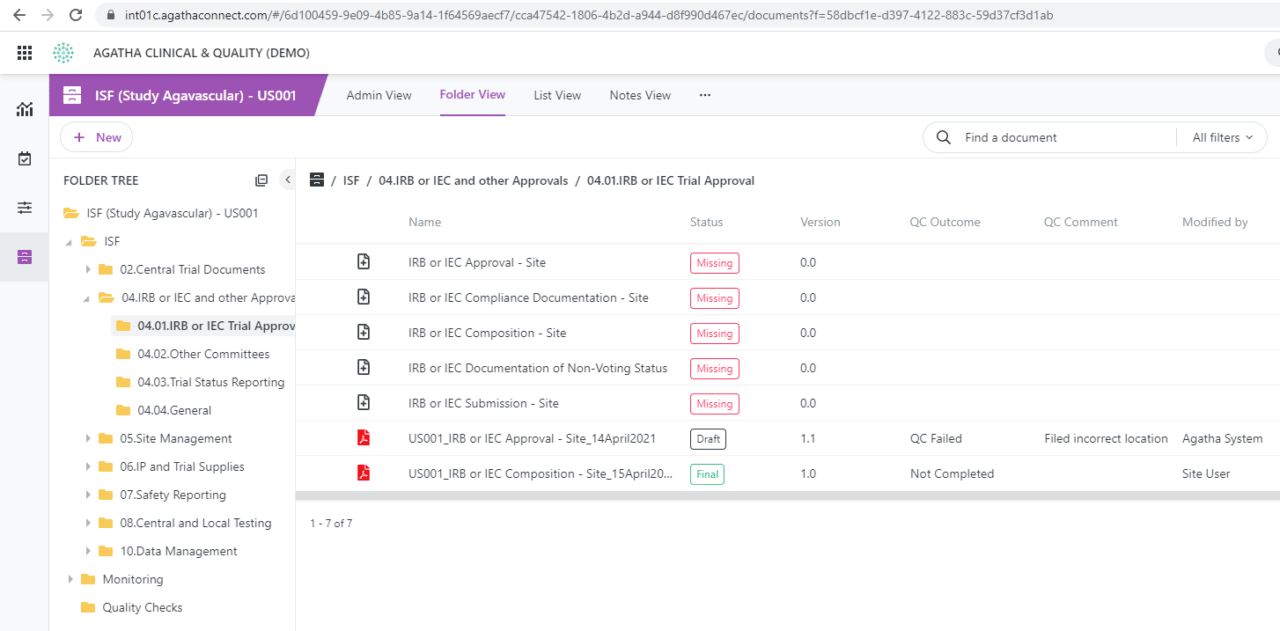
Share binder contents (investigator site files, or eISF) in real time among monitors, sites, and sponsors. This enables remote document review and quality check in your clinical trial process, crucial for maintaining a steady flow of documents between sites. Utilize forms to communicate monitor notes and assign tasks to site personnel.
Pinpoint documents for the quality check process and track the quality review using custom views. Take advantage of workflows with electronic signatures to meet all compliance requirements. Establish automated workflows to transition final clinical study documents to the Master TMF.
Our eISF solution offers a secure document exchange platform that aligns with documentation requirements and trial conduct standards, boosting your trial oversight capabilities.
Ready do dive in?
For inquiries about our products and services, or to sign up for a demo, click here.