Improve CRO and Sponsor Collaboration with a Shared eTMF

Traditional thinking says that an electronic trial master file application (eTMF) is simply a repository for storing documents related to a clinical study. But today, that’s only partly correct. A modern eTMF application provides so much more to the clinical operations teams. And one of these things is improved collaboration between the sponsor and CRO (contract research organization).
To understand the relationship between sponsor and CRO and how that relates to the eTMF, Agatha joined customer DZS Clinical Services for a webinar that provided insights into ensuring a strong relationship of collaboration and communication.
Here are some highlights from that webinar (and a link to the replay at the end).
The Relationship Between Clinical Study Stakeholders
Kari Brown, Senior Director of Clinical Services for DZS, shared her insights on the relationship between CRO and sponsor. She said that the best working relationship sees the CRO as an extension of the complete team, where opinions and expertise are respected, and mutual trust is built on clear upfront communication and expectations. Kari offered her five hallmarks of a great CRO-sponsor relationship:
- Clear expectations upfront. For example, provide detailed roles and responsibilities for those on the CRO side, review the SOW and talk through it to ensure everyone is on the same page.
- Mutual respect for the expertise that each party brings to the table. Kari said the client (sponsor) knows their drug/device in-depth, and CROs can improve its services by tapping into that and truly understanding the product and the history.
- Open communications. Things come up; mistakes happen, Kari said. So honest, open communications are critical at all times. Also, coming to the table with solutions or having corrective or preventative actions already established for when things don’t go as planned is essential. She also said that it’s important to do lessons learned on an ongoing basis, not just at the end.
- Collaboration. It’s a collaborative effort even if the sponsor assigns many responsibilities to the CRO, Kari said. The CRO will always need the sponsor’s input.
- Work with a shared purpose. Take the time to understand what the goal is. What are we working for? Working with a shared purpose means everyone has a stake in the process and wants to see it go successfully.
When talking about collaboration, you need to talk about technology. The eTMF is only one type of technology required. The slide below shows some other technologies used.

Who’s TMF is it, Anyway?
The sponsor owns and has ultimate responsibility for the TMF. But they don’t always use their own eTMF. Sometimes, the sponsor supplies the eTMF and has its IT team set up a study and provide access to the CRO. Other times, the sponsor wants the CRO to supply the eTMF application. In either case, it is a collaborative effort to manage the TMF.
Kari said that some sponsors delegate all the management of the TMF, but she argued that the sponsor still needs to have a role. For example, the sponsor can do overall quality control (QC) of the TMF. Essentially, said Kari, the sponsor must know their TMF, so having them involved is very important.
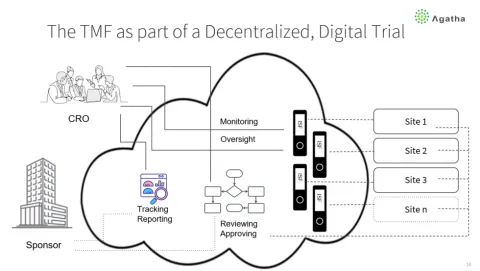
Role of the eTMF in the Digital Decentralized Trial Process
The eTMF is not just a document repository. It is expected to tell the story of the clinical trial, and that is becoming increasingly important because the FDA auditors are more and more requesting access to the eTMF to perform their audits. Sometimes, said Kari, they aren’t even speaking to the sponsor or the CRO involved.
Ken said that the eTMF is often at the center of conversations, activities, and processes between the sponsor, CRO, Sites, and regulatory agencies. When it’s at the center, it’s not only about managing documents but also about communications and collaboration.
You can also extend it to the Site side to monitor and perform quality processes on the investigator site file (ISF). For example, a CRA can look at essential documents within the ISF and transition them to the TMF as they are reviewed and approved. Or the CRA can add comments and feedback on a document to get the Site to make modifications.
According to Kari, the eTMF is a powerful tool for collaboration:
- Review plans and get comments and feedback within the system.
- Track activities through auditing and version control.
- It helps with accountability (tasks, due dates, etc.).
Supports monitoring of the ISF.
The eTMF acts like a Hub for the digital decentralized trial process. If that’s true, then what does the eTMF need to have for features and capabilities?
There is a basic set of features that most eTMF vendor provide. But in addition to basic features, other critical capabilities make it the hub for the management of the TMF:
- Integration with each Site’s ISF for oversight, monitoring, and quality assurance.
- Document tracking and reporting for sponsors (% complete, reviews and approvals), taking management out of email or fax.
- Collaborative authoring of documents
- Task assignment
- Dashboards for reporting and QA.
This isn’t your father’s eTMF
“This isn’t just a space to drag and drop things in a folder. This is a system to manage relationships and interactions and support the trusted environment so that there are fewer dropped balls and there are less of those human errors that you were talking about, Kari. In my view, this goes a long way towards improved quality, as well, when we have a system that captures all [the majority] of the interactions.”
Ken Lownie, Head of North American Operations, Agatha
Watch the full replay to hear Kari’s stories working in a CRO with multiple sponsors and learn more about eTMF applications. You can also download the whitepaper: Beyond Storage: How CROs and Sponsors Work Together to Manage the Complexities of eTMF Oversight.
Ready do dive in?
For inquiries about our products and services, or to sign up for a demo, click here.
