Creation, Storage and Management of Regulatory Documents
How we help
Agatha Reguratory Benefits
-
 Streamline Global Regulatory Compliance with AgathaOur regulatory document management software offers centralized data management, automated processes, and visibility into regulatory activity, aligning with your business objectives. With Agatha’s regulatory submission software, manage your global operations more effectively, lower non-compliance risks, and speed up product development.
Streamline Global Regulatory Compliance with AgathaOur regulatory document management software offers centralized data management, automated processes, and visibility into regulatory activity, aligning with your business objectives. With Agatha’s regulatory submission software, manage your global operations more effectively, lower non-compliance risks, and speed up product development. -
 Accelerate regulatory approval with an eRegulatory solutionObtaining regulatory authorization is the most critical step in bringing new drugs, medical devices, and therapies to market. Success means patients gain faster access to new options, while mistakes lead to delays that cost time and money. A regulatory management application is essential to streamline and accelerate all regulatory activities and processes.
Accelerate regulatory approval with an eRegulatory solutionObtaining regulatory authorization is the most critical step in bringing new drugs, medical devices, and therapies to market. Success means patients gain faster access to new options, while mistakes lead to delays that cost time and money. A regulatory management application is essential to streamline and accelerate all regulatory activities and processes. -
 Ensure FDA compliance with regulatory information management softwareUse the application to collect, organize, and manage regulatory documents from all clinical trial sites in your regulatory binders before submission, providing a single, authoritative source for all required content.
Ensure FDA compliance with regulatory information management softwareUse the application to collect, organize, and manage regulatory documents from all clinical trial sites in your regulatory binders before submission, providing a single, authoritative source for all required content.

Mablink Bioscience Manages Regulatory Compliance with Agatha
Main Features
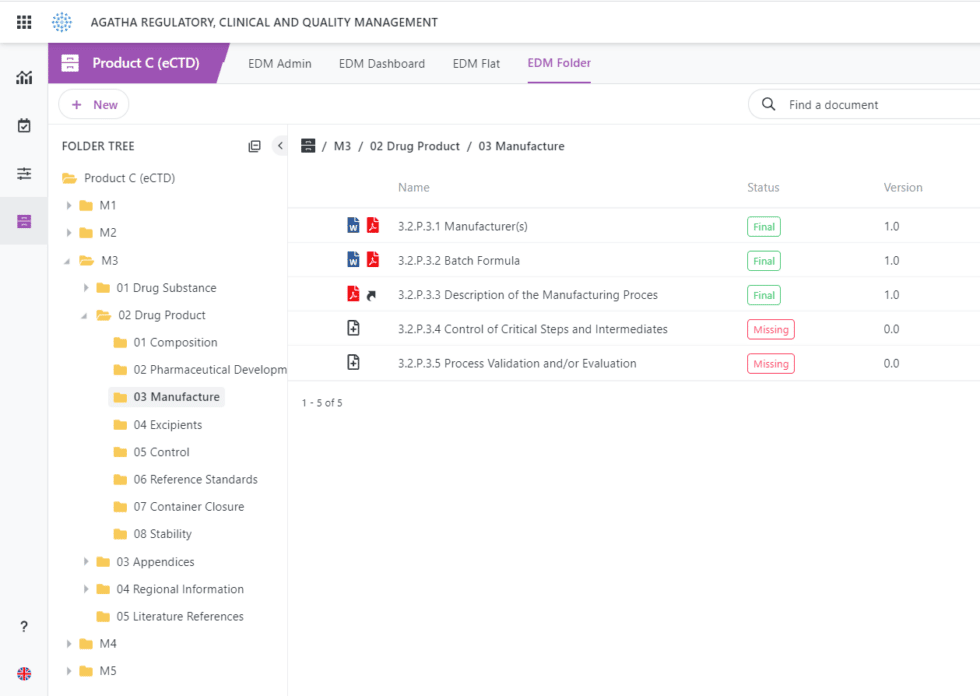
Browse submission archives in the application using the submission viewer.
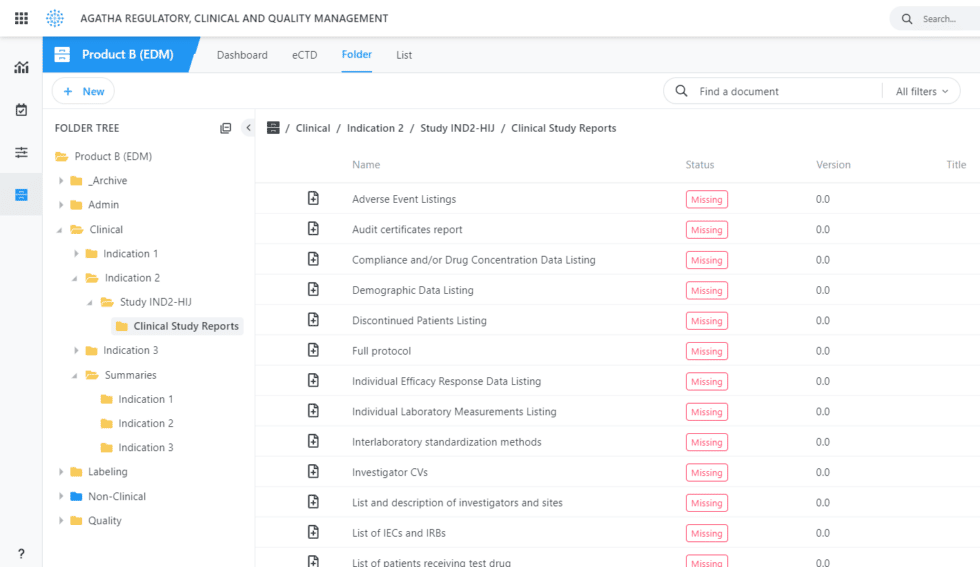
Out-of-the-Box integration with most eCTD submission software products.
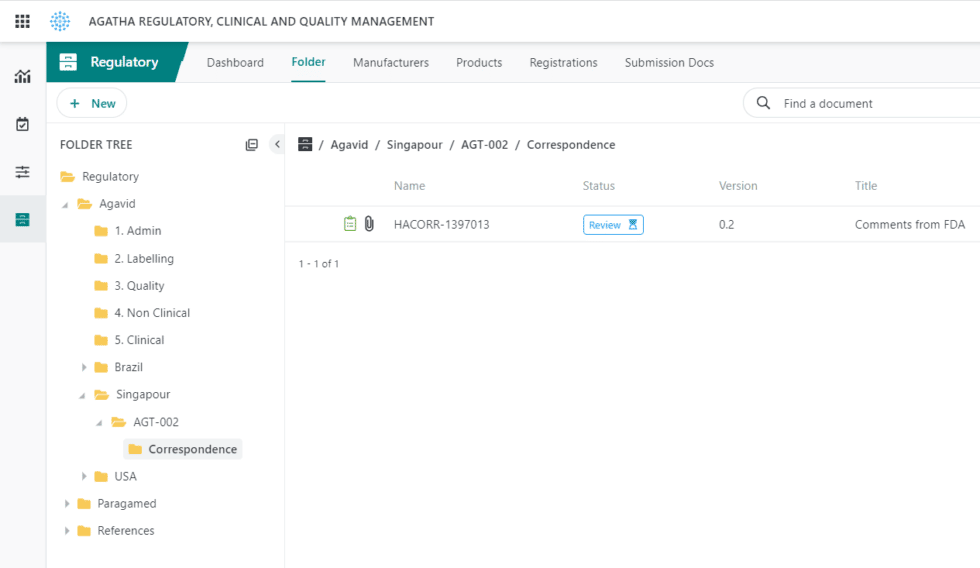
Track regulatory applications and health authority correspondence.
Ready do dive in?
For inquiries about our products and services, or to sign up for a demo, click here.


