Agatha Regulatory – Regulatory information management system for clinical research
eRegulatory application for clinical research
A complete, centralized regulatory management application for collecting, coordinating and managing all the information and documentation required for regulatory submissions. Choose Agatha’s platform for your regulatory documents.
Accelerate regulatory approval with an eRegulatory solution
Obtaining regulatory approval is the single most important step in bringing new drugs, devices, and therapies to market. Success means patients get new options sooner, while mistakes mean delays that waste time and money. A regulation management application is a must-have to accelerate all regulatory activities and regulatory processes.
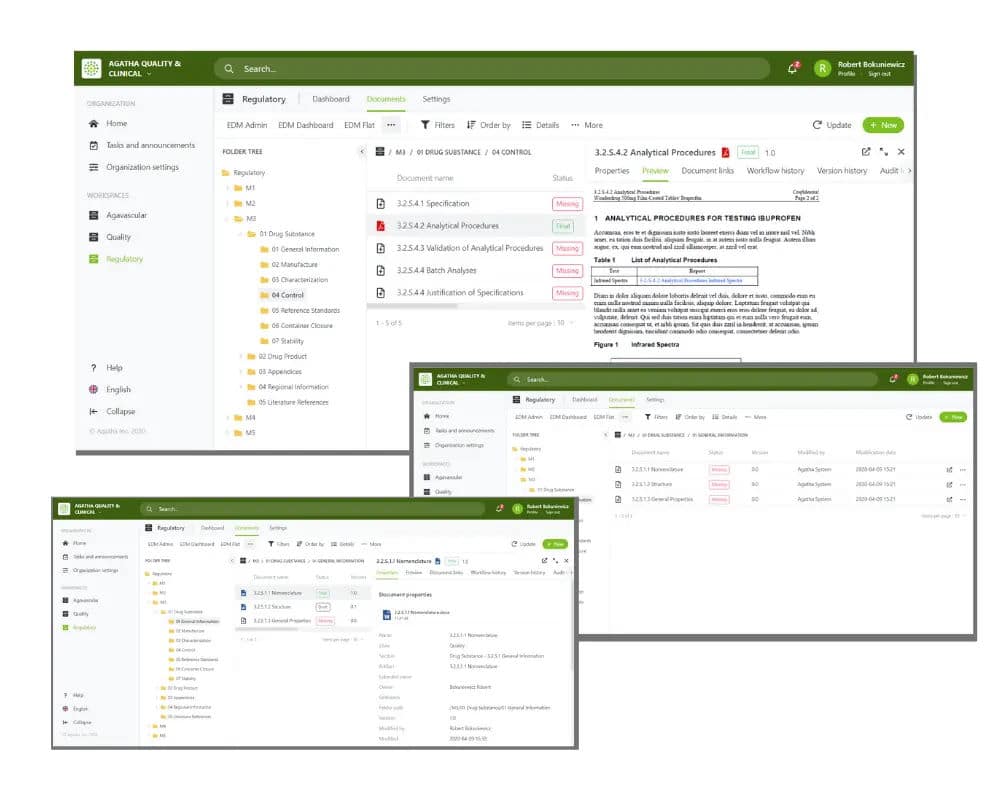
Choose efficient regulatory process management with Agatha Regulatory
eRegulatory Solution for pharmaceutical companies
Agatha Regulatory is a comprehensive, ready-to-use regulatory management application that addresses the challenge of creating a unified set of submission documents from content that is often created in many places and by different teams, sometimes located in many countries.
Ensure FDA compliance with a regulatory information management software
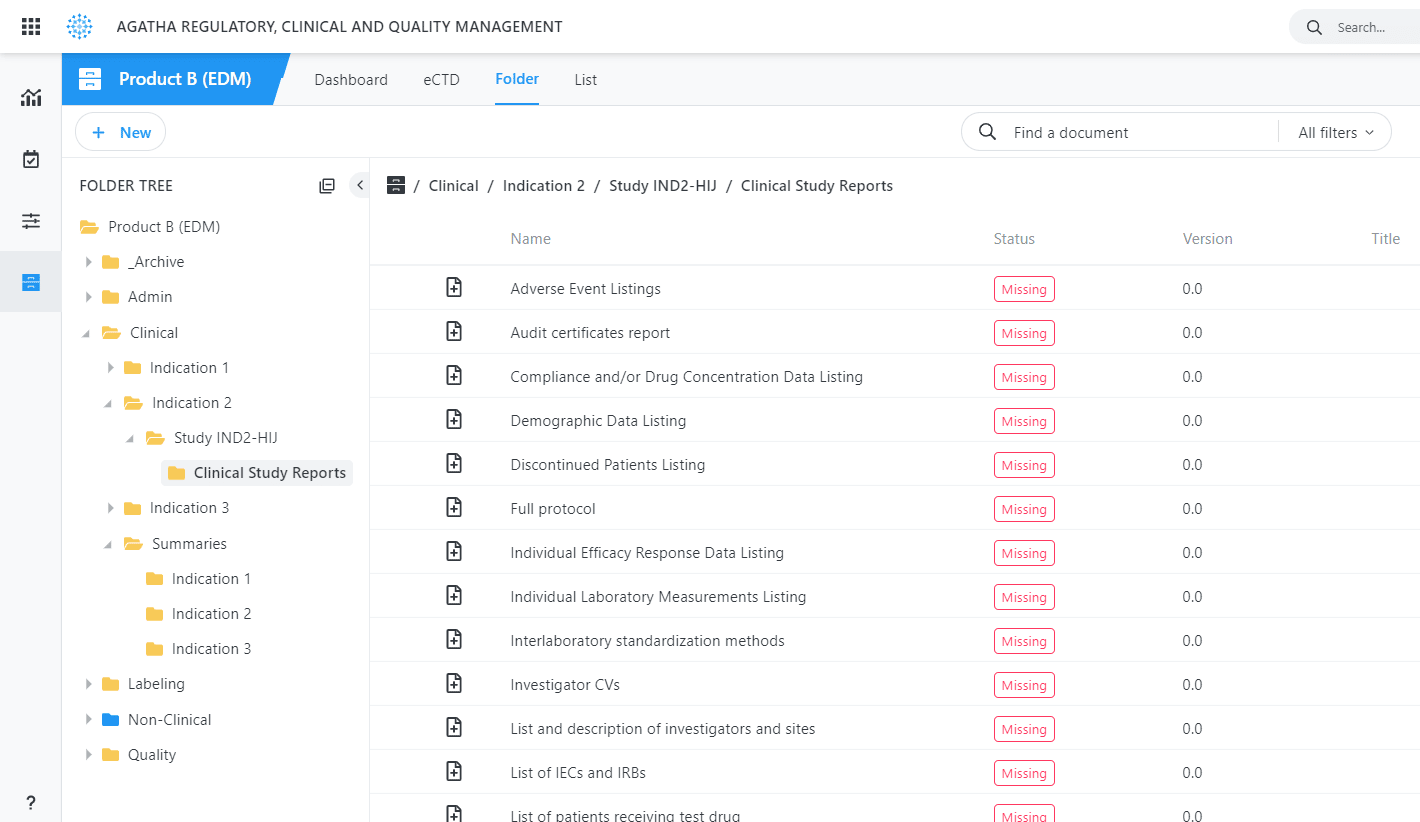
Use the application to collect, organize, and manage regulatory documents from all clinical trial sites in your regulatory binders prior to submission, providing a single and authoritative source for all required content.
Ensure regulatory approval with Agatha’s eRegulatory solution

Kiyuki Tanifuji
Group manager Development Planning Group, Clinical Development, Nihon Medi-Physics Co., Ltd.
How Agatha’s regulatory information management system accelerates the submission process
View Submissions
Browse submission archives in the application using the submission viewer.
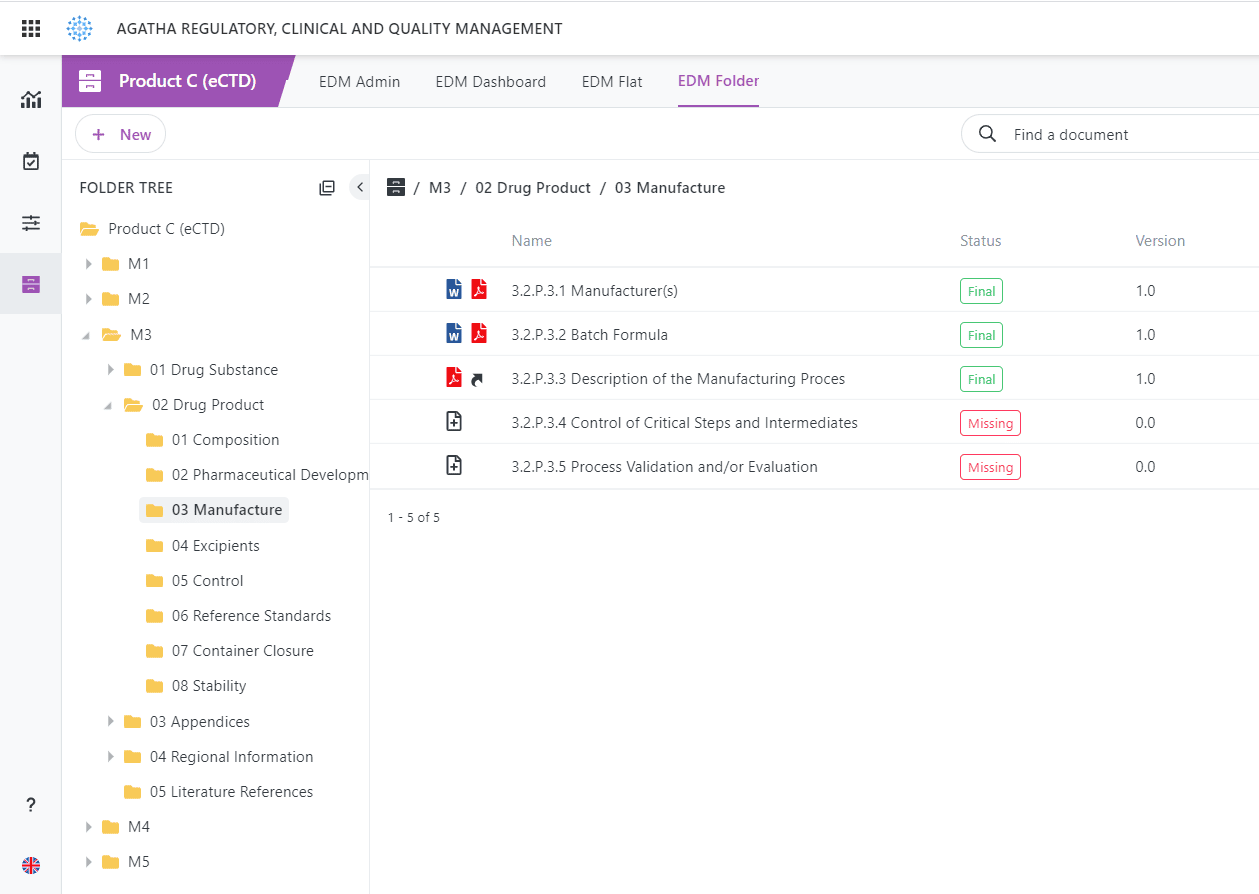
eCTD Integration
Out-of-the-Box integration with most eCTD submission software products.
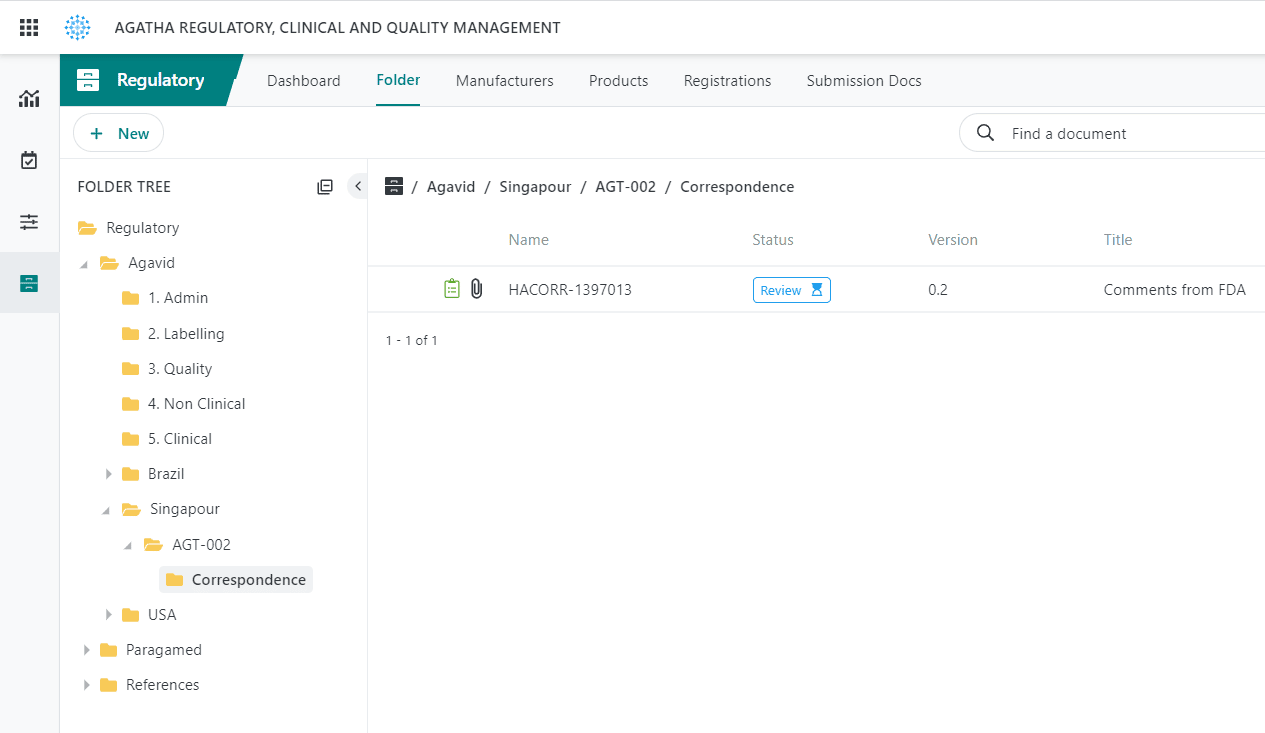
Track Applications
Track regulatory applications and health authority correspondence.
The best way to know if an Agatha Application meets your needs is to try it out. And good news – we offer a free trial of our eRegulatory solution for your clinical trials.
A Few More Agatha Regulatory Features
Based on Standards
Agatha Regulatory is based on the standard EDM Reference Model.
Annotations
Simultaneous online review of documents with shared annotations.
What’s Missing
Identify missing sumbmission items automatically at any point in the process.
Learn more about regulatory compliance and Agatha’s regulatory information management system
What is a regulatory information management software?
A regulatory information management system (RIM system) is a software that helps companies accelerate their regulatory compliance processes and ensure compliance to various regulatory authorities. In general terms, this category of information management system helps pharmaceutical companies manage their submissions to regulatory authorities.
eRegulatory solutions such as Agatha Regulatory offer a centralized platform for companies to manage various regulatory documents and prepare standardized submission documents in regulated formats.
The importance of regulatory compliance in the pharmaceutical industry
Regulatory changes have resulted in an increasing number of requirements being placed on companies operating within the pharmaceutical industry. These requirements can range from simple documentation to complex processes that require significant resources to implement. In addition to these requirements, companies must ensure that they remain compliant with any future changes to existing legislation.
What are regulatory challenges faced by pharmaceutical businesses?
Larger and smaller companies often face similar challenges in their regulatory workflows. Many feel these regulatory process issues acutely at a certain inflection point – an increasing product line, due date for submissions, or geographical expansion. Two common challenges faced by pharmaceutical businesses during the development process are evolving regulatory requirements and high volumes of documentation.
Evolving FDA regulatory requirements
In a pharmaceutical development firm, regulatory documentation can no longer be viewed as a discrete task. Staying compliant to shifting regulatory requirements through clinical trials forces businesses to continuously adjust to proliferating information resources, variable quality and heterogeneous information. Many departments and teams require access to regulatory documents, and the costs of being non-compliant are significant for pharmaceutical businesses and potential users. There lacks a holistic view of regulatory documentation throughout the entire development process.
The volume of documentation required to stay compliant
The sheer volume of paperwork involved in regulatory compliance is challenging for pharmaceutical businesses. As many businesses handled thousands for documents and forms, even minor changes to documentation, labels, statistical analyses and so forth create ripples throughout the system, causes confusion and are time consuming. In addition, as many different departments and groups use regulatory documentation, there is a need for organization-wide standardization and efficiency.
What are the advantages offered by a RIM system?
A regulatory information management system offers central data management, ensuring that timely and accurate regulatory knowledge is readily available and consistently applied across the organization. Automated processes eliminate manual steps, allowing teams to save time and focus on higher value tasks. Visibility into regulatory activity allows clinical trial site teams to better understand how regulatory timelines align with business objectives.
Regulatory efficiency and effectiveness mean lower risks for companies regarding non-compliance or having to remove products from markets. Stronger, more confident global regulatory compliance means they can better manage their operations globally. Faster product development allows them to bring new products to market faster.
What should pharmaceutical companies look for in an eRegulatory software?
eRegulatory technology exists to reduce the time to market for new pharmaceutical products or medical devices. With the goal of saving time and increasing collaboration and efficiency, eRegulatory solutions like Agatha Regulatory allow life sciences companies to reduce the costs associated with non-compliance before, during and after clinical research.
In order to stay compliant to regulatory requirements and save time, life sciences companies should look for a eRegulatory solution that offers the following features:
- Audit trails;
- Version control;
- Training;
- Security controls;
- eSignature capabilities;
- Standard naming and filing conventions;
- Supports remote monitoring;
Tasks performed by Agatha Regulatory
Agatha’s eRegulatory application:
- Identifies the products required for international regulatory agencies;
- Collects technical documents such as Electronic Common Technical Documents (eCTDs);
- Controls the access to documents when dealing with different products and regulatory agencies;
- Manages changes and revisions to documents and products;
- Generates compliant submission documents;
- Enables electronic signatures of study documents;
- Issues and track submissions across the paper and through the appropriate gateways;
- Minimizes effort and calendar time for companies to replicate regulatory submissions between products and regulatory authorities;
- Ensures that original content is submitted on time and correctly;
- Fulfills regulatory requirement for electronic equipment, such as 21 CFR Part 11;
What is the difference between regulation and compliance?
Although they are closely related, it is important to consider the differences between regulation and compliance.
Regulation by regulatory bodies like the FDA
Regulations are a set of rules and standards imposed by a regulatory agency such as the FDA, usually in order to protect people or the planet from any potential risks or damages.
To ensure that its products are safe for use by consumers, companies in the pharmaceutical and medical industry must comply with the regulations set forth in the Food and Drug Administration’s (FDA) 21 CFR Part 11 legislation. This ensures that the company maintains the quality of its products throughout their lifecycle and ensures that they are safe for consumer use.
Compliance
Regulatory compliance is an ongoing process followed by pharmaceutical companies to meet the requirements of regulatory bodies of their market. Being compliant is not binary. Rather, specific processes and activities undertaken by staff are either compliant or not. Most life-science organizations are also governed by multiple regulatory regimes, ranging from those that relate to product manufacturing to those that pertain to workplace health and safety. Therefore, a life science organization might be in compliance with one set of regulations yet out of compliance with another.