Biotech QMS: A unique quality management system for the biotech and pharmaceutical industry
Every industry and discipline has unique quality management requirements and frameworks. That’s why a generic Quality Management System (QMS) is not a good fit when addressing the requirements (like GMP or GCP) of a biotech or medical device company. Choose a Biotech QMS made specifically for biotech and pharmaceutical businesses.
Experience the ultimate Biotech QMS solution
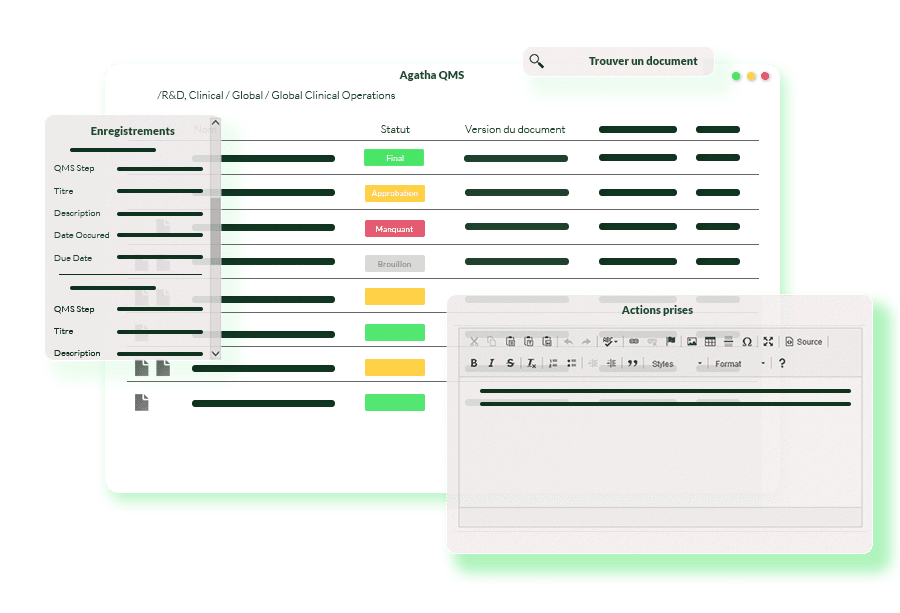
Agatha Quality: The Biotech QMS solution for efficient development and product safety
Agatha Quality helps biotech organizations ensure product quality and meet customer requirements while maintaining comprehensive records of all events and activities related to their manufacturing process. Our Biotech QMS offers a single platform for all your compliance needs, from validations to audit trails, regulatory inspections, and preventative actions.
How can our Biotech QMS help your company achieve customer satisfaction?
As a biotech organization, you face constant regulatory requirements from agencies like the FDA and EMA. Our Quality Management System (QMS) is specifically designed for the biotech industry and offers a comprehensive platform for compliance, quality assurance, and continuous improvement, allowing you to exceed customer expectations.
The best way to know if our Biotech QMS meets your needs is to try it out. And good news – we offer Biotech businesses a free trial for Agatha Quality.
Comprehensive quality management for the biotech industry
Our Biotech QMS includes a wide range of processes and practices, such as audit management, inspection management, and periodic reviews, ensuring your compliance with regulatory requirements. The QMS also features a document management system to maintain accurate records of your quality management activities, decisions, and quality issues.
Powerful insights for continuous improvement
With Agatha Quality, our QMS includes powerful tools for continuous improvement, such as data analysis and corrective action planning, allowing you to identify areas for improvement and implement changes that enhance efficiency, product safety, and customer satisfaction.
Adaptable and user-friendly Biotech QMS
Our QMS is adaptable to your specific needs and requirements. We understand that every biotech company is unique, which is why our Biotech QMS solution is user-friendly and easy to navigate. Our quality team is always available to provide training and support.
Management review and periodic reviews for a robust Biotech QMS
Implement Agatha Quality, and watch your biotech company thrive. With our QMS, you can ensure product quality, meet regulatory requirements, and exceed customer expectations. Our QMS is designed to help you achieve your quality goals and objectives, improve your quality operations and processes, and promote quality assurance throughout your organization.
Agatha Quality: A Biotech QMS for the Life Sciences
Effectively managing clinical, compliance, and quality processes is a mission-critical task at life sciences companies. Quality improvements accelerate trials and improve the results of clinical studies, while quality failures can result in delays, added costs, and major disruptions. Agatha Quality is the foundation of quality for your ClinOps and GMP program, providing a complete, ready-to-use Biotech QMS solution.
Discover Agatha Quality and our Biotech QMS
Learn more about Agatha Quality, our clinical quality management system, and how it can benefit biotech organizations by providing improved patient safety, guaranteed regulatory compliance, and proactive quality assurance throughout the research process.

Risk-based monitoring and quality system
Our Biotech QMS solution for enterprises is an end-to-end quality management system that combines forms, frameworks, documents, and workflows, offering a complete toolset for quality managers. Agatha QMS is preconfigured, validated, and ready-to-use but easy to adapt to specific process requirements.
How Agatha Quality supports compliance in Biotech
Advanced forms
Work with ready-to-use standard forms and processes, or adapt them to support your needs. Our Biotech QMS solution includes rich text fields that allow the addition of images and formatted text. Use the form designer to update the look and framework of your forms.
Complete metrics and reporting for your quality team
Get all the reporting you need with built-in dashboards and reports. Generate custom reports and export any report to Excel or a generic CSV format. Cross Workspace Reporting allows you to create views and reports across workspaces and export results to Excel for dashboard reporting.
Inspection-ready and fully compliant Biotech QMS
Create complete quality records with electronic signatures, recorded approvals, and audit trails, all ready for inspection. Agatha QMS is fully compliant with 21 CFR Part 11 and EU regulatory requirements. Our quality management application is also fully integrated with other Agatha applications, making it easy to create references between them (e.g., a CAPA can reference an SOP).
A biotech QMS solution that is more than just an online record
QMS forms in Agatha can be converted to PDF at any point, resulting in an appropriately formatted, signed electronic record.
Biotech businesses get more with Agatha Quality
Basic preview and annotations
Preview and annotate PDF and Office documents, and images in Agatha Quality’s report form.
Advanced integration toolkit
Use Agatha’s Rest API to allow applications to upload or export items from Agatha (includes documentation and sample code).
An end-to-end process
A complete, closed-loop process captured in an expanding form, from initial issue to preventative action.
Full compliance
A validated system that is compliant with GxP requirements, EU regulations, and FDA 21 CFR Part 11.
Learn more about Agatha Quality and our Biotech QMS Solution
What is a biotech quality management system?
It is a type of clinical quality management system (CQMS), a software application used for quality monitoring during clinical research by pharmaceutical and biotech companies. Clinical research requires a Biotech QMS, as a well-designed clinical quality management system will help ensure that all procedures, processes, protocols, documents, etc., that occur throughout the entire course of a clinical study are managed effectively and audited for quality.
How can Agatha's Biotech QMS benefit biotech organizations?
With the assistance of a clinical QMS like Agatha’s Biotech QMS solution, an organization operating in the biotechnology segment of the healthcare industry will be able to implement the applicable regulatory standards, concepts, and methodologies needed to achieve the goals of clinical research.
QMS biotech applications like Agatha Quality also play an important role in keeping clinical research compliant with regulatory requirements by international regulatory agencies, such as Good Clinical Practices (GCP), the European Medicines Agency (EMA), and the US Food and Drug Administration (FDA), and others.
What are the advantages of a clinical Biotech QMS solution?
Improved patient and consumer safety
A clinical Biotech QMS generates reliable data that an organization, its partners, regulatory and healthcare authorities, and other stakeholders can use for monitoring and improving clinical products and the lives of patients. Using a Biotech QMS solution such as Agatha Quality gives clinical trial participants and end users of the results of clinical research the assurance that medications/treatments that they receive were developed using proper research.
Guaranteed regulatory compliance throughout the research process
Managing quality through a central QMS biotech application ensures regulatory compliance during the entire clinical trial process. This risk mitigation will include first-in-human studies to post-marketing surveillance of new pharmaceutical products.
Improved clinical research quality management
Reduce or eliminate repetitive quality issues during clinical studies such as unintentional non-compliance, insufficient staff training, insufficient supervision by management, lack of protocol clarity, and insufficient quality control in the collection and recording of data threatening patient safety/data integrity.
Agatha Quality’s clinical Biotech QMS solution offers a strong organizational structure to help pharmaceutical and biotech businesses systematically improve the overall performance of their clinical research, accomplish their quality control and training goals, and complete clinical trials efficiently.
Proactive approach to quality assurance
A reactive approach is not enough for a quality management software (CQMS): good risk management practices are essential. Proactively managing clinical quality with a well-designed Biotech QMS solution instead of reacting to issues related to investigational products when they occur avoids wasting valuable time and resources.
What are regulatory requirements for drug development and clinical studies in the pharmaceutical industry?
The main regulatory requirements for any project of clinical research or clinical investigation conducted by a biotech organization are:
Good clinical practice (GCP)
“Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording, and reporting trials that involve the participation of human subjects. Compliance with this standard provides public assurance that the rights, safety, and well-being of trial subjects are protected, consistent with the principles that have their origin in the Declaration of Helsinki, and that the clinical trial data are credible.The objective of this ICH GCP
Guideline is to provide a unified standard for the European Union (EU), Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions.
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).”
ISO 14155:2020
The ISO 14155:2020 “specifies general requirements intended to:
- protect the rights, safety and well-being of human subjects,
- ensure the scientific conduct of the clinical investigation and the credibility of the clinical investigation results,
- define the responsibilities of the sponsor and principal investigator, and
- assist sponsors, investigators, ethics committees, regulatory authorities and other bodies involved in the conformity assessment of medical devices.”
European Medicines Agency clinical trials regulation
Regulation (EU) No 536/2014 states that:
“In a clinical trial the rights, safety, dignity, and well-being of subjects should be protected and the data generated should be reliable and robust. The interests of the subjects should always take priority over all other interests.”
What are the main elements of a clinical Biotech QMS?
The core elements of an effective digital quality strategy in a Biotech QMS solution involve the following elements:
- Processes
- Resources
- Roles and responsibilities
- Partnering
- Risk management
- Issue management
- Knowledge management
- Documentation that supports achieving quality
Choose Agatha Quality for a comprehensive, efficient, and user-friendly Biotech QMS solution that will help your biotech organization stay ahead of the competition and ensure product safety, development, and customer satisfaction.