Clinical Operations Software – Discover Agatha’s ClinOps software
If you are managing clinical trials, we know a few things about you. You are smart, extremely competent, and really (REALLY) busy. And you know what you want in a ClinOps application: you want complete, effective, ready-to-use applications that manage TMF and site-side ISF content that ensure compliance and inspection readiness.
Let Agatha’s ClinOps software help you.
Connect, collaborate, and control your clinical studies.
Regulatory and financial requirements, as well as the geographical dispersion of participating sites, make managing clinical studies with paper, spreadsheets, and email extremely difficult. There are too many items, in too many places to manage and track effectively.
Agatha’s clinical operations software centralizes all your clinops
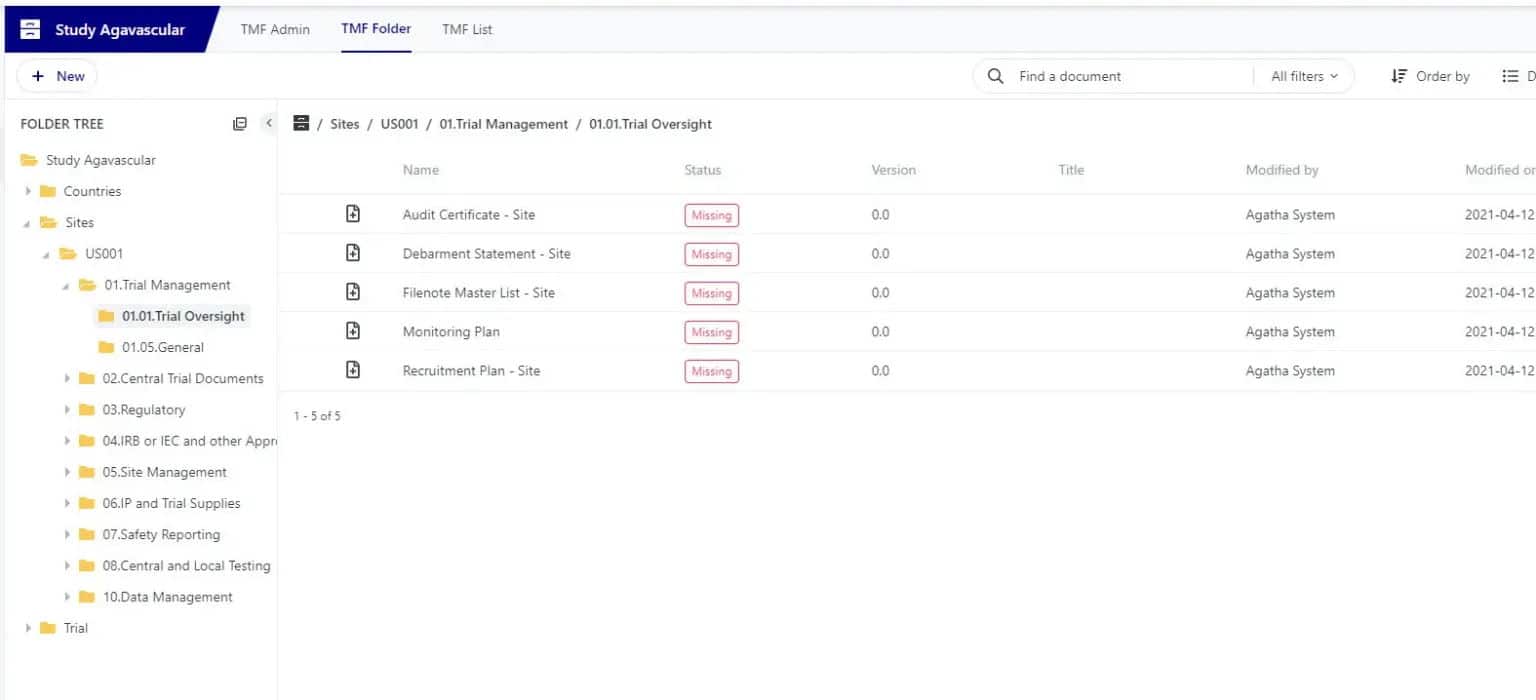
Agatha’s ClinOps applications ensure efficient study operations and complete regulatory compliance. Agatha Clinical eTMF and Agatha Remote ISF provide:
- Automated information sharing between sponsors, CROs, and sites
- The creation and management of a Trial Master File, ready for inspection at all times
- The ability to track a wide range of clinical trial information
- Complete reporting and dashboard capabilities
And best of all, Agatha’s applications are cloud-based, ready-to-use applications that can be in production in a matter of weeks.
Collaborate efficiently
Share automatically
Minimize risks
Report on all information
Collaborate efficiently
Share automatically
Minimize risks
Report on all information
Accelerate your clinical studies with the right clinical operations software
Consolidated data for decision making
ClinOps seamlessly integrates with a wide variety of third party applications and vendors so that you don’t need to go out of your way to get data analytics into the system. It eliminates the need for manual entry of data by allowing you to pull it directly from these sources.
Short ClinOps software implementation time
We’ll meet with you to discuss your project. Afterward, we’ll configure ClinOps Pro for your particular situation. You don’t need any outside consultants or technical support.
Customizable according to specific clinical needs
ClinOps Pro is customizable to fit your specific needs, whether you’re using a CRO or running your own operations. We can also help you salvage any manual tracking information you’ve already collected.
Dashboards and trackers to help you shorten the time between when someone signs up for your product. With the help of external data, your ClinOps Pro system provides visual tools with only the relevant insight needed for critical and efficient decisions and risk management.
Reduced workload thanks to process automation
ClinOps Pro includes an import feature and automation tools so that you don’t need to spend hours manually importing files and creating integrations. With these features, you get a clinical operations team with more free time to focus on high-quality work.
Enhanced collaboration directly through Agatha
Don’t just get insights; also monitor, comment, follow up and assign tasks – all in one place. Instead of trying to force people into unnatural ways of working, let them use their existing tools and processes.

« The decision to move to an electronic TMF and move its management in-house was the right decision for us. With Agatha’s help, we were able to configure an eTMF application that supports our needs today and can adapt with us as our processes evolve.»
Senior Clinical Research Associate
InCarda Therapeutics
Agath Applications for Clinical Operations Leaders
Agatha Clinical eTMF
Ensure every critical document is accounted for with a complete eTMF application. With Agatha Clinical you can be sure that all required documents you need for each site and study are present and everything is inspection ready.
Interested in seeing how Agatha’s applications can help you improve your clinical and quality processes? Take one for a test drive.
Interested in seeing how Agatha’s applications can help you improve your clinical and quality processes? Take one for a test drive.
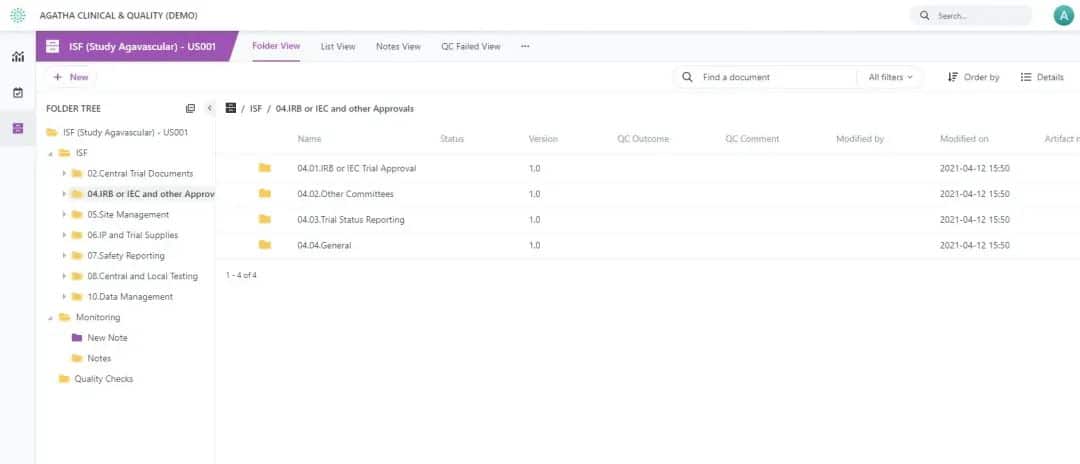
Agatha Remote ISF
Are you looking for a way to reduce on-site monitoring visits, yet still have the ability to monitor and inspect clinical research sites remotely? Agatha Remote ISF (Investigator Site File) provides Sites with the ability to upload critical documents securely and enables remote monitoring and inspection.
Learn more about Clinops and Agatha’s clinical operations software
What is ClinOps software?
Clinical trials are an essential part of the healthcare industry and Agatha’s industry experts have been working to develop a single platform to drive trial efficiencies, improve quality monitoring and reduce overall cost.
Clinical operations software is a system used to facilitate communications between clinical operations teams, clinical trial sponsors, clinical trial processes, and clinical trial teams to monitor product development, user issues, quality management and clinical trial performances. They provide decision support tools for critical business decisions and facilitate issue management and collaboration between stakeholders.
Why use Agatha as your clinical operations software?
Agatha’s platform helps to reduce monitoring visit time, improve study start-up by providing shared study start-up workflows, reduce reconciliation time and provide real-time inspection readiness. Clinical trial software solutions have been integrated into the platform to enable clinical operations teams to manage clinical trial processes, clinical trial data, and clinical trial training. It also provides clinical process automation and cross-functional business processes for the life sciences industry.
Agatha’s platform provides a centralized source for trial execution, clinical trial documentation, and clinical trial software solutions. It also helps to reduce operational costs, improve clinical trial processes, and provide a comprehensive solution for patient care. With its ability to provide real-time visibility and decision support tools, the platform helps to drive efficiencies and reduce costs for the healthcare industry.
What is the meaning of ClinOps?
ClinOps, or Clinical Operations, refers to the process within pharmaceutical and medical device companies that governs the development and execution of clinical trials. This involves professionals using Clinical Trial Management Systems to handle study design, resource allocation, adherence to timelines, and day-to-day operations, all while maintaining an emphasis on quality patient care.
What does a clinical operations team do?
A clinical operations team manages the planning and execution of clinical trials in an organization. They are responsible for study design, enrollment, managing trial activities at clinical sites, and ensuring regulatory requirements are met. This team coordinates with contract research organizations, manages study participant care, and employs Clinical Trial Management Systems for real-time data capture.
What is the difference between clinical development and clinical operations?
Clinical development in pharmaceutical companies involves the creation of clinical trial protocols, study design, and enrollment of patients for trials. Clinical operations, on the other hand, refer to the practices and efforts involved in the day-to-day operations of executing these trials, ensuring standards are met, and maintaining compliance with regulatory bodies.
What are the goals of clinical operations?
Clinical operations aim to efficiently manage resources and timelines in conducting clinical trials, maintaining high-quality care for study participants, ensuring compliance with regulatory requirements, and streamlining clinical trial operations. This involves working with clinical trial managers, clinical research organizations, and utilizing Clinical Trial Management Systems effectively.
What are the duties of a clinical operations coordinator?
A clinical operations coordinator is responsible for organizing study activities, coordinating with clinical teams, and overseeing clinical trial protocols. They ensure efficient resource allocation, handle patient visits, manage electronic documents, and adhere to regulatory standards. Coordinators are also responsible for managing the electronic trial master file and maintaining clear and accurate entries in Clinical Trial Management Systems.
What are examples of clinical goals?
Clinical goals can include efficient patient enrollment, maintaining high standards in patient care, ensuring timely completion of trials, and adherence to regulatory requirements. In terms of clinical trial designs, a goal could be developing a study design that effectively measures the outcomes of a drug or device under test.
What are 3 common types of goals?
In a clinical trial context, three common types of goals include: improving patient care, maintaining compliance with regulatory authorities, and efficiently managing trial supply. These goals are typically set by clinical trial managers and are achieved through organized planning, proper resource allocation, and efficient trial management practices.
What is an example of a clinical SMART goal?
A clinical SMART goal could be: “Implement a Clinical Trial Management Platform in the next 6 months to enhance real-time data capture, streamline clinical trial process, and improve communication among clinical teams, thereby improving efficiency in our clinical operations.”
What is a smart goal in clinical practice?
A SMART goal in clinical practice is a specific, measurable, achievable, relevant, and time-bound objective. For instance, “Increase patient enrollment by 25% in the next quarter by enhancing our outreach efforts and optimizing the trial application process.”
What are the roles in clinical operations?
Roles in clinical operations range from clinical trial managers responsible for overall trial supply management and clinical teams caring for patients, to professionals overseeing the clinical trial management platform and managing entries into Clinical Trial Management Systems. All these roles contribute to maintaining day-to-day operations at study sites and executing study activities.
Is clinical operations a good field?
Absolutely. Clinical operations is a vital field in the healthcare industry. It offers the opportunity to work on the development of life-saving drugs and treatments, interfaces with multiple disciplines including sciences and regulatory authorities, and plays a key role in the planning and execution of clinical trials.
What is the job description of head of clinical operations?
The head of clinical operations oversees the structure and function of the clinical operations department within a pharmaceutical or medical device company. They manage the development and execution of clinical trials, ensure regulatory compliance, maintain relationships with contract research organizations, and supervise resource allocation. Furthermore, they oversee clinical trial managers, ensure quality care for study participants, and make strategic decisions about trial design and implementation.
What is the difference between clinical development and clinical operations?
Clinical development is primarily concerned with the conceptualization and design of clinical trials, which includes creating clinical trial protocols, planning the trial, and enrolling patients. On the other hand, clinical operations involve the execution of these plans, which includes managing trial activities, ensuring adherence to timelines and regulatory standards, managing resources, and handling day-to-day operations.
What is the difference between clinical and operational?
Clinical activities primarily involve direct patient care, such as patient visits, administering treatments, and collecting data from patient diaries. Operational activities, on the other hand, revolve around the management and administrative aspects of these clinical functions. This includes managing resources, coordinating trial activities, ensuring regulatory compliance, managing trial supply, and handling report forms. These operational tasks are critical in supporting the oversight of clinical aspects of a trial and are facilitated by Clinical Trial Management Systems.
What types of trials are managed by study managers?
Study managers oversee various types of trials including randomized control trials, observational studies, and decentralized trials. These professionals are integral to the clinical trial process, planning the development and execution of these studies, and monitoring clinical trial outcomes.
How important is building relationships in the role of clinical ops leaders?
Building relationships is essential for clinical ops leaders. This involves nurturing professional ties with contract research organizations, regulatory bodies, and teams within the organization, including clinical trial managers, coordinators, and researchers. Strong relationships foster collaboration, streamline the clinical trial process, and can lead to improved clinical trial outcomes.
What is the role of decentralized trials in clinical operations?
Decentralized trials, also known as remote or virtual trials, are an emerging type of clinical trial where study procedures are conducted at a distance, often at the participant’s home. In the role of a study manager, managing decentralized trials can enhance patient recruitment and retention, reduce trial timelines, and improve clinical trial outcomes.
What is a development plan in clinical operations?
A development plan in clinical operations outlines the strategic approach for conducting a clinical trial. This can include the types of trials to be conducted, the process for managing those trials, and methods to measure clinical trial outcomes. Development plans are crucial for study managers and clinical ops leaders as they guide the direction of clinical operations.
How do clinical ops leaders contribute to positive clinical trial outcomes?
Clinical ops leaders play a critical role in ensuring positive clinical trial outcomes. Their leadership and strategic planning in the clinical trial process, management of various types of trials including decentralized trials, and focus on building strong relationships can significantly enhance the effectiveness of trials. Furthermore, their role in devising and implementing the development plan can lead to improved results in clinical operations.