Agatha Regulatory – Leading regulatory submission software & document management solution
Regulatory document management software for clinical research
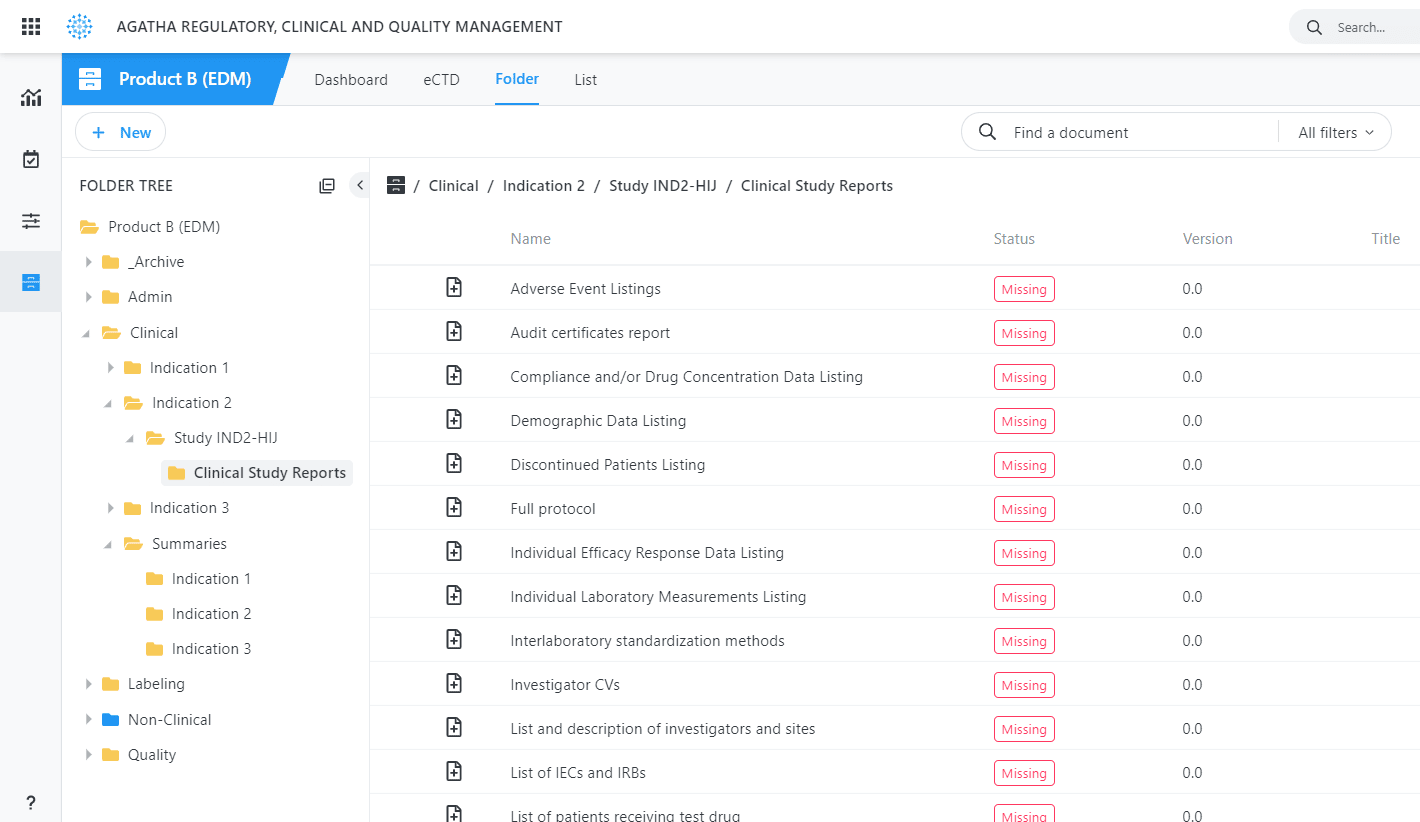
Agatha’s eRegulatory application stands as a regulatory document management software powerhouse, efficiently collating and managing all necessary information for regulatory submissions. Transform your regulatory operation with Agatha’s regulatory submission software.
Unlock efficient regulatory processes with our regulatory submission software now
Revolutionize your regulatory approval process with our regulatory submission software
Achieving regulatory approval is the cornerstone for marketing new drugs, devices, and therapies. Ensure success with our regulatory submission software – a critical tool in expediting regulatory operations and processes, and avoiding costly errors.
Regulatory submission software for streamlined regulatory process management
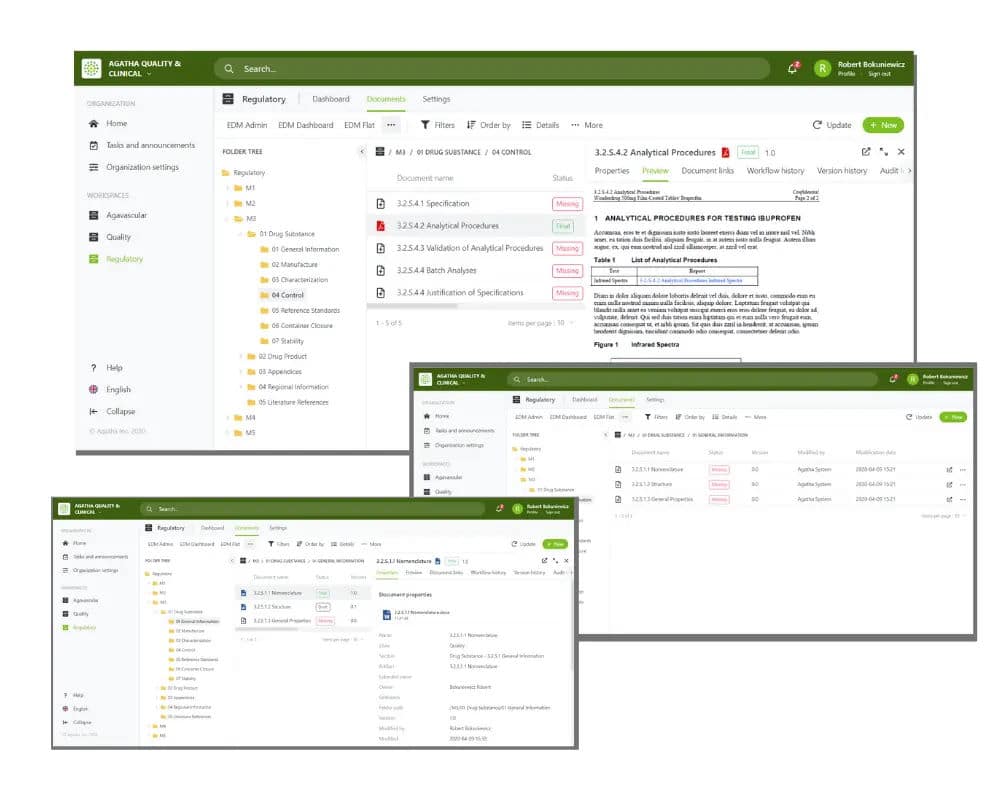
Agatha’s regulatory submission software, designed for pharmaceutical companies, provides an all-encompassing regulatory document management software solution. It efficiently compiles submission content, even when created by different teams across various locations.
Choose efficient regulatory process management with Agatha Regulatory
Agatha’s regulatory document management software: Your path to FDA compliance
Use our regulatory document management software to gather and manage regulatory documents from all clinical trial sites. Prioritize regulatory review and submission with our organized and comprehensive software solution.
Mitigate risks with Agatha’s regulatory submission software

Kiyuki Tanifuji
Group manager Development Planning Group, Clinical Development, Nihon Medi-Physics Co., Ltd.
Agatha’s regulatory submission software: Driving the submission process forward
Experience our regulatory submission software features, such as tracking applications, streamlining eCTD submissions, and integrating with other software products. Take advantage of our free trial for your clinical trials to discover the power of our regulatory document management software.
Unlock efficient regulatory processes with our regulatory submission software now
Agatha’s regulatory document management software: Going above and beyond industry standards
Our regulatory document management software offers unique features, such as simultaneous online document review and automatic identification of missing submission items, elevating the quality of your dossier management and eliminating manual processes
How Agatha’s regulatory information management system accelerates the submission process
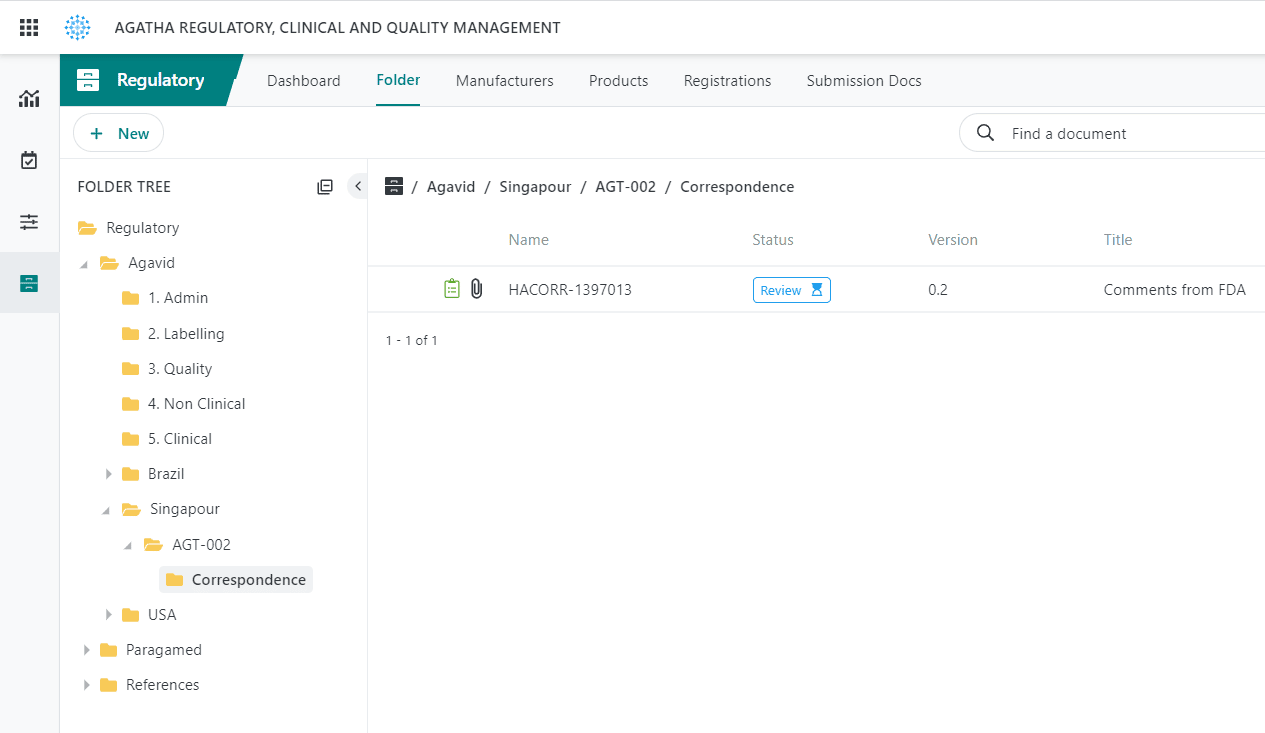
View Submissions
Browse submission archives in the application using the submission viewer.
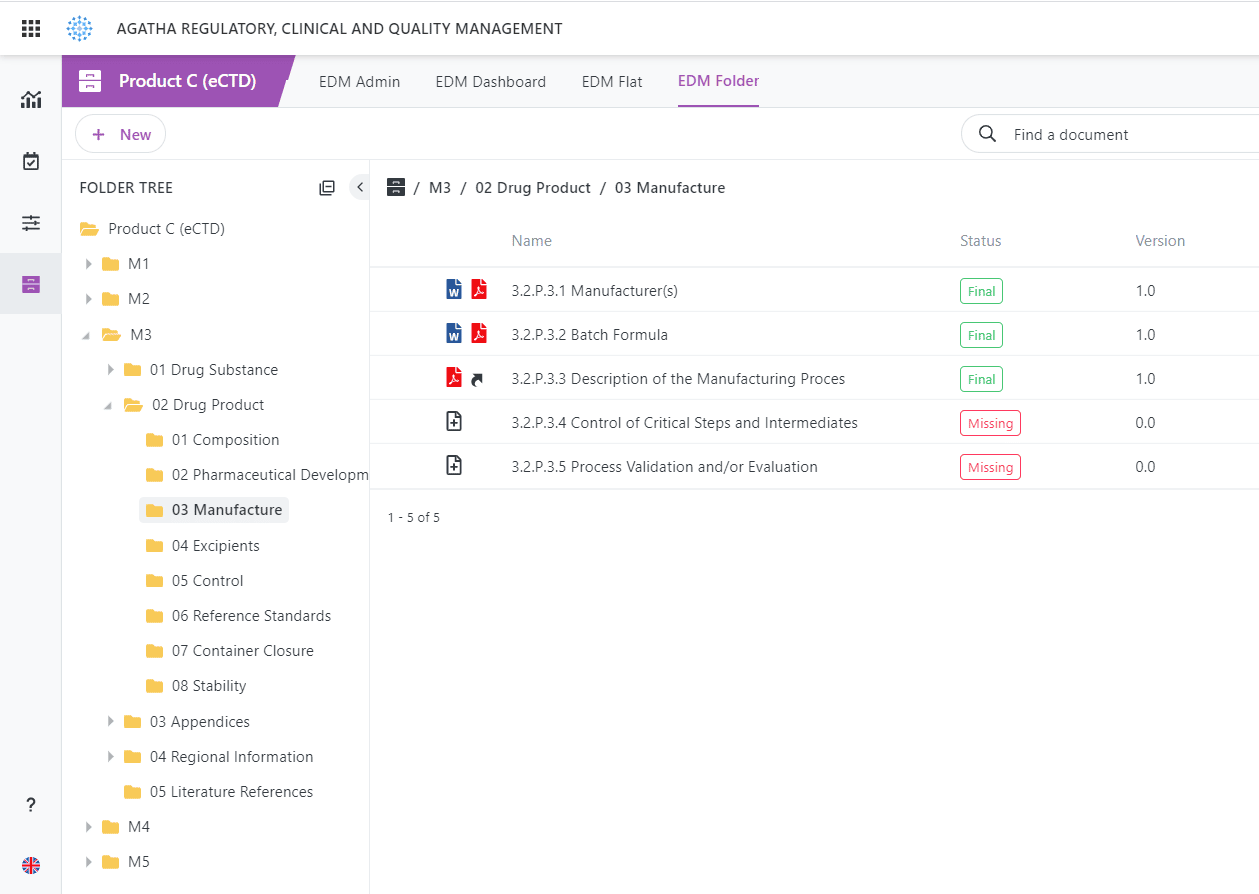
eCTD Integration
Out-of-the-Box integration with most eCTD submission software products.
Track Applications
Track regulatory applications and health authority correspondence.
The role of regulatory document management software in regulatory compliance
A regulatory information management system (RIM system), such as Agatha’s regulatory document management software, accelerates regulatory compliance processes and ensures compliance with regulatory bodies. It offers a unified platform for managing regulatory documents and preparing standardized submission documents in regulated formats.
Jumpstart your regulatory approval with our regulatory submission software

The Importance of regulatory compliance in the pharmaceutical industry: Agatha’s regulatory submission software solution
Increasing regulatory changes demand more from pharmaceutical companies. From simple documentation to complex processes, Agatha’s regulatory submission software helps companies to meet these requirements and stay prepared for future legislative changes.
Addressing regulatory challenges with Agatha’s Regulatory Document Management Software
Pharmaceutical companies often face challenges in their regulatory workflows. Our regulatory document management software offers a systematic solution to overcome these challenges, ensuring compliance, mitigating risks, and enabling smoother regulatory operations.
Unlock efficient regulatory processes with our regulatory submission software now
Advantages of Agatha’s Regulatory document management software
Our regulatory document management software offers centralized data management, automated processes, and visibility into regulatory activity, aligning with your business objectives. With Agatha’s regulatory submission software, manage your global operations more effectively, lower non-compliance risks, and speed up product development.
Learn more about regulatory compliance and Agatha’s regulatory submission software
What should pharmaceutical companies look for in an eRegulatory software?
eRegulatory technology exists to reduce the time to market for new pharmaceutical products or medical devices. With the goal of saving time and increasing collaboration and efficiency, eRegulatory solutions like Agatha Regulatory allow life sciences companies to reduce the costs associated with non-compliance before, during and after clinical research.
In order to stay compliant to regulatory requirements and save time, life sciences companies should look for a eRegulatory solution that offers the following features:
- Audit trails;
- Version control;
- Training;
- Security controls;
- eSignature capabilities;
- Standard naming and filing conventions;
- Supports remote monitoring;
Tasks performed by Agatha Regulatory
Agatha’s eRegulatory application:
- Identifies the products required for international regulatory agencies;
- Collects technical documents such as Electronic Common Technical Documents (eCTDs);
- Controls the access to documents when dealing with different products and regulatory agencies;
- Manages changes and revisions to documents and products;
- Generates compliant submission documents;
- Enables electronic signatures of study documents;
- Issues and track submissions across the paper and through the appropriate gateways;
- Minimizes effort and calendar time for companies to replicate regulatory submissions between products and regulatory authorities;
- Ensures that original content is submitted on time and correctly;
- Fulfills regulatory requirement for electronic equipment, such as 21 CFR Part 11;
What is the difference between regulation and compliance?
Although they are closely related, it is important to consider the differences between regulation and compliance.
Regulation by regulatory bodies like the FDA
Regulations are a set of rules and standards imposed by a regulatory agency such as the FDA, usually in order to protect people or the planet from any potential risks or damages.
To ensure that its products are safe for use by consumers, companies in the pharmaceutical and medical industry must comply with the regulations set forth in the Food and Drug Administration’s (FDA) 21 CFR Part 11 legislation. This ensures that the company maintains the quality of its products throughout their lifecycle and ensures that they are safe for consumer use.
Compliance
Regulatory compliance is an ongoing process followed by pharmaceutical companies to meet the requirements of regulatory bodies of their market. Being compliant is not binary. Rather, specific processes and activities undertaken by staff are either compliant or not. Most life-science organizations are also governed by multiple regulatory regimes, ranging from those that relate to product manufacturing to those that pertain to workplace health and safety. Therefore, a life science organization might be in compliance with one set of regulations yet out of compliance with another.
What is a regulatory submission?
A regulatory submission is a formal communication made to health authorities, such as the FDA, containing documents necessary for product approval. Regulatory submission management tools are essential for this process, ensuring that all electronic submissions meet the required standards.
What are eCTD software used for?
A regulatory submission is a formal communication made to health authorities, such as the FDA, containing documents necessary for product approval. Regulatory submission management tools are essential for this process, ensuring that all electronic submissions meet the required standards.
How much is eCTD software?
The cost of eCTD software varies depending on its features, the number of users, and the level of customer support provided. Validation processes and the flexibility of electronic submissions functionality can also affect the price.
What is eCTD publishing?
eCTD publishing involves compiling, validating, and submitting regulatory documents in the eCTD format, a standard required by many health authorities. Effective eCTD publishing necessitates submission tools that ensure documents meet all regulatory requirements.
What is CTD vs eCTD?
CTD (Common Technical Document) is the format used for regulatory submissions before the advent of eCTD (Electronic Common Technical Document). eCTD submissions are the electronic version of CTD, offering a more streamlined and efficient process for validating and managing regulatory documents.
What are 5 modules in eCTD?
The five modules in eCTD are: 1) Administrative Information and Prescribing Information, 2) Common Technical Document Summaries, 3) Quality, 4) Nonclinical Study Reports, and 5) Clinical Study Reports. These modules organize information in eCTD submissions, enabling easier review by health authorities.
What is regulatory document management system?
A regulatory document management system is a tool that facilitates the creation, validation, and management of regulatory documents. Such systems improve the efficiency of regulatory submission management, ensuring compliance with health authorities’ requirements for electronic submissions.
What is compliance document management?
Compliance document management involves maintaining, organizing, and validating documents to ensure regulatory compliance. Effective compliance document management requires submission tools that support validation processes and enable seamless electronic submissions.