When dealing with Regulatory Authorities there is no Room for Error.
If you lead a Regulatory Affairs team in a life sciences company, you are keenly focused on making sure that every interaction with the Regulatory Authorities is perfect. Every communication is planned. Every submission is complete. And every interaction is documented. You need an application designed specifically for tracking and storing every communication between your organization and the Authority. We have one for you.
Track every interaction.
In regulatory affairs, no surprises is the motto. Every submission has to be planned to a “T” and executed with precision. Any mistake can result in delays that cost not just time and money, but lives.
Agatha Regulatory is an application that is already configured to let you plan each interaction, and track it through completion. Critical dates are recorded and notifications sent to everyone involved. Communications are logged and stored. And submission content is drafted, reviewed, approved and tracked right through archiving. All in one easy-to-use application.
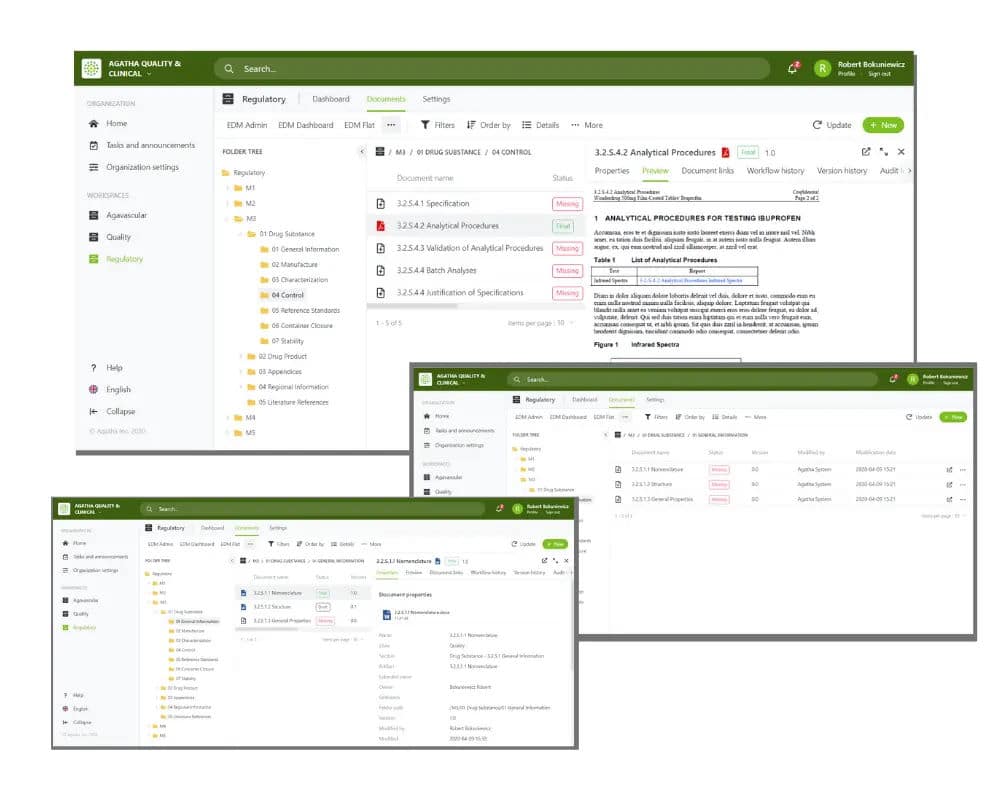
Track regulatory applications and health authority correspondence. Follows EDM Reference Model.
Out-of-the-Box integration with most eCTD submission software products.
Browse submission archives in the application using the submission viewer.
Simultaneous online review of documents with shared annotations.
The best way to find out if the Agatha apps meet your needs is to try them out. And the good news is you can try them today.
Applications for regulatory affairs professionals
Agatha Regulatory
Agatha Regulatory is a comprehensive, ready-to-use regulation management application that addresses the challenge of creating a unified set of submission documents from content that is often created in many locations, sometimes in many countries. Use the application to collect, organize, and manage regulatory documents prior to submission, providing a single and authoritative source for all required content.