Learn the difference between clinical quality management and manufacturing quality management.


Learn the difference between clinical quality management and manufacturing quality management.

Boston, MA, USA; Lyon, France; Tokyo, Japan. June 10, 2021, Agatha Inc. announced new features and capabilities for its clinical, quality, and compliance applications for life sciences companies. A total of 11 new capabilities were announced, focused on...

New UI Delivers High Performance and Accelerates Adoption Tokyo, Japan; Lyon, France; Boston, MA, USA. February 25, 2021. Agatha Inc. announces the official release of its new PRISM User Interface for the Agatha Suite of Clinical Operations and Quality...
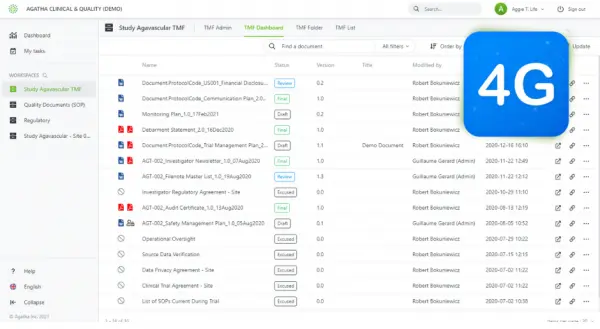
Electronic trial master file solutions have gone through several evolutions from complex, highly customized, on-premises platforms to custom off-the-shelf solutions, and all the way to the latest evolution - cloud-based, easy-to-use applications. We are now seeing...

Electronic systems for managing clinical study documentation emerged in the 1990s and have evolved through three generations. One of these applications is the electronic trial master file (eTMF). Who needs an eTMF and why use it? Read on to find out. Defining the...

If you are in the market for an electronic Trial Master File system (eTMF solution), it is almost certain you have made a list of requirements that leads with compliance, functionality, ease-of-use, and cost. And if I had my way, I would stop you right there, on...
Sponsors and CROs have invested millions of dollars or more in automating clinical operations since the 90s. And every study they do is includes one or more clinical sites where patients are treated, and data originates. Yet, clinical sites trail behind in terms of...

At the Recent Clinical Quality Oversight Forum, I had a chance to give a presentation entitled “The Path to Successful Remote Monitoring (watch it on-demand).” For me, it was something of a culmination of all the research, interviews, and thinking I have done this...

The topic of remote monitoring of clinical trials has gotten enormous attention since February of this year, as travel to clinical sites became difficult and, in some cases, impossible. Whether at conferences (virtual, of course) or on-line, ClinOps leaders are...

Jill Heinz, Director of Clinical Research, shares her insights on clinical sites and the value of clinical operations technology.